Navi Medical Technologies is pleased to announce that the US Food and Drug Administration (FDA) has granted Breakthrough Device Designation (BDD) for the Neonav® ECG Tip Location System as a method for navigating and positioning umbilical venous catheters in newborn patients.
The Breakthrough Device Designation is only given to devices that represent a breakthrough in effective treatment or diagnosis of life-threatening or debilitating conditions, and to technology where there is no approved or cleared alternative currently marketed in the US.
Navi COO Shing Yue Sheung is excited about how the designation will support the Neonav® regulatory submission process, “we are thrilled to be given the opportunity to work closely with the FDA to develop the Neonav® device in order to help raise the level of care for newborns, who are one of the most vulnerable patient groups that are often overlooked in new device developments.”
One of the primary benefits availability to Navi through the Breakthrough Device Designation is a priority review pathway with the FDA, which includes the opportunity to receive additional review resources to expedite patient access to the Neonav device.
“While the Neonav® System is not yet available in the US market, we are hopeful that Breakthrough Device Designation will help expedite patient access to the Neonav® System and provide clinicians with a real-time catheter placement and surveillance solution, as well as further highlight the significant unmet needs facing newborn patients,” says Shing.
The Breakthrough Device Designation was supported by early stage Neonav® clinical studies conducted at the Royal Women’s Hospital in Melbourne, Australia, as well as other well documented published studies that highlight the adverse events associated with malpositioned umbilical venous catheters, and other central lines, used in the care of newborns.
“Unfortunately, progress in catheter placement technology for critically-ill newborns has been limited over the years, with the current standard of care for tip placement in newborns still relying on X ray confirmation that takes place after the procedure has been completed. X rays’ lack of real-time feedback often results in delays to treatment, repeated dosage of ionising radiation, and offers very little in terms of surveillance. While other ECG-based technologies do exist, they are primarily for older children and adults, do not have surveillance capabilities built in, and cannot easily or effectively be used to treat the unique requirements of critically-ill newborns.”
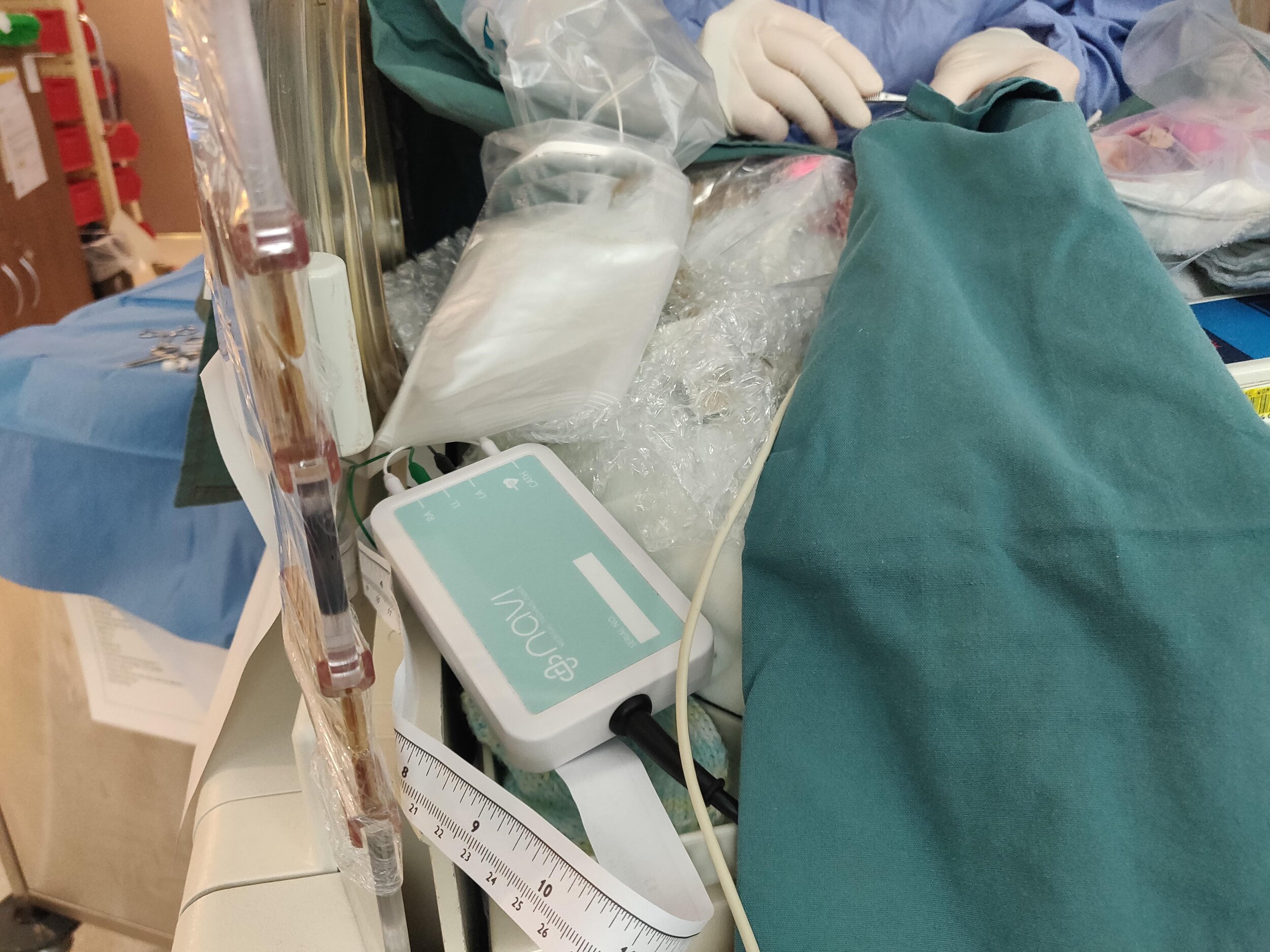
The Neonav® ECG Tip Location system offers a number of important improvements over the current standard of care for placing and monitoring central catheter placements in critically-ill newborn patients. The Neonav®’s new ECG-based algorithm technology will provide real-time feedback to clinicians on central catheter tip location inside newborn patients, as well as provide fast and effective surveillance capabilities to help detect instances of catheter migration or dislocation.
ABOUT CENTRAL LINE CATHETERS
Over 1 million central lines are placed in newborn and pediatric patients globally, allowing for fast and effective delivery of mediations, nutrients and fluids. Limited real-time visibility of the catheter during insertion, as well as throughout the dwell time, can lead to instances of movement and misplacement that can result in adverse events, complications, increased costs and in some cases even death.
ABOUT THE NEONAV® ECG TIP LOCATION SYSTEM
The Neonav ECG Tip Location System is currently being developed to address the unique needs to safely place central lines in critically-ill newborn and pediatric patients. The Neonav® system utilises a novel proprietary algorithm that will enable fast and accurate location detection of central lines during the initial insertion procedure, and will enable ongoing surveillance to alert clinical users when a catheter moves to an unsafe location.