The team at Navi is pleased to announce that, in partnership with the Royal Women’s Hospital, we have been successful in receiving grant funding through the recent round from the Victorian Medical Research Accelerating Fund (VMRAF).
Video: Introduction to the Neonav® ECG Tip Location device concept
The research project being funded, will be conducted at the Royal Women’s Hospital in Melbourne, and use Ultrasound technology to help further validate the accuracy of the Neonav® algorithm, as well as the use of ECG technology to accurately detect central venous catheter migration in critically-ill newborns.
Associate Professor Christiane Theda, Navi’s Chief Medical Officer, co-founder, and lead investigator for the research project, is excited about commencing this next round of research;
“The clinical and product teams at Navi have worked hard over the past few years to develop a first clinical prototype of our Neonav® device which has performed brilliantly in our studies so far. This next phase of work will allow us to collect important data that will give us new insights into how we can better refine and improve our technology so it can help us in our goal of providing safer care for critically-ill newborns.”
Pictured left to right: Shing Yue Sheung, Mubin Yousuf, A/Prof. Christiane Theda, Wei Sue, Alex Newton
Launched by the Victorian Government in 2017, the VMRAF provides grant support to accelerate health and medical research and fast-track innovative projects from research to real-world impact. This is the second VMRAF grant that Navi has been successful with, after also receiving support in 2020 for a Round 4 project.
Navi CEO and co-founder Alex Newton says these sort of grant programs are an important part of developing local medtech industries in Melbourne;
“We’re so grateful for the support from the Victorian government. Grants such as the VMRAF are critical sources of funding for early-stage medical device companies looking to develop products for a global market.”
In 2022, Navi has received support from various grant programs including the VMRAF, MMCP and TAIP funds form the Victorian Government, as well as the Federal Governments Accelerating Commercialisation Grant.
To date, Navi has secured over $2 million in State and Federal government funding, which has played a key role in furthering the development of Neonav® device.
These programs provide important validation on both the commercial and clinical strategy, and enables Navi to establish research, development and manufacturing capabilities locally in Victoria.
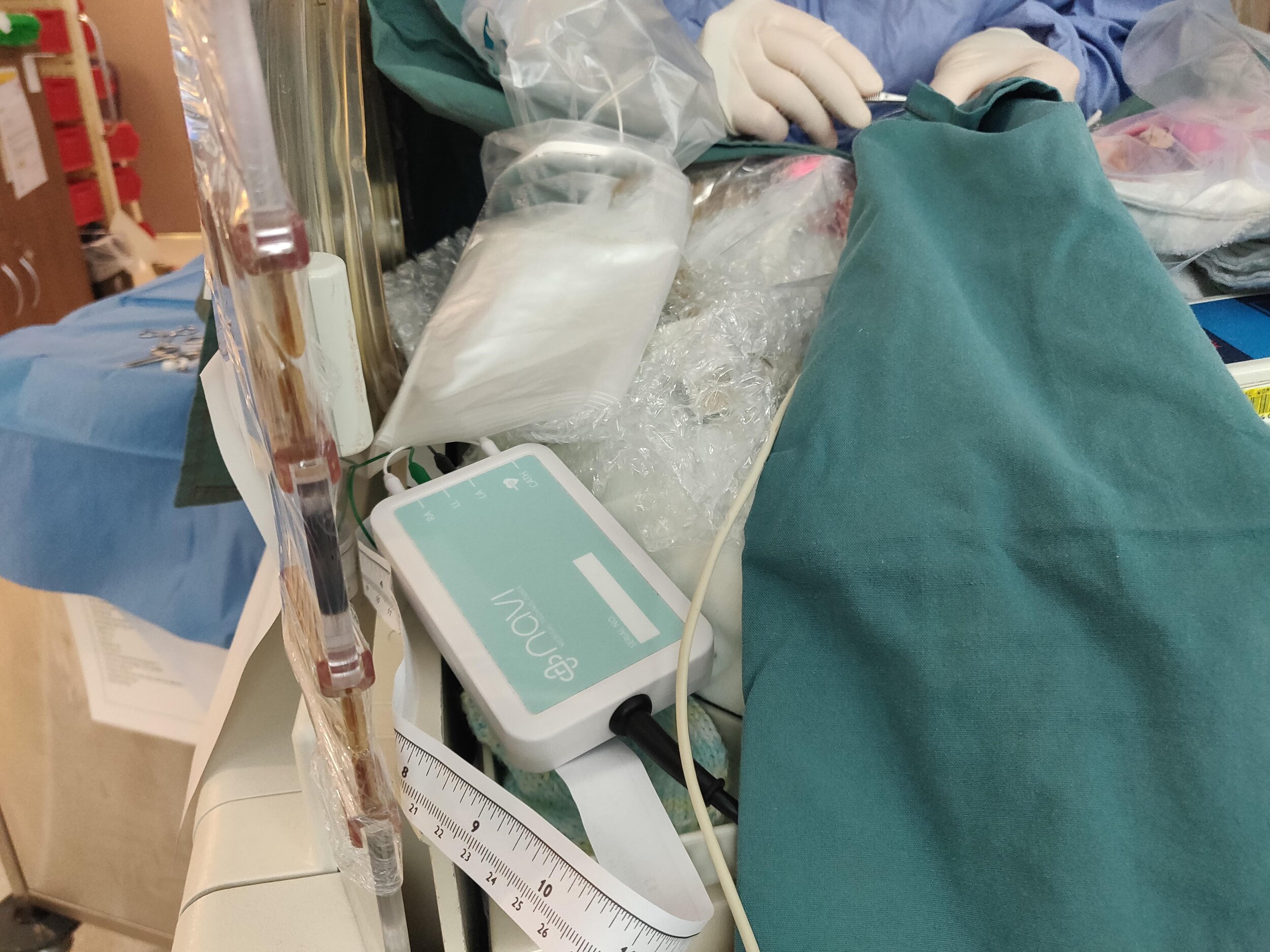
Pictured: Early-stage Neonav clinical prototype used in part of a study at the Royal Women’s Hospital in Melbourne, Victoria
The Navi team would like to extend a special thank you to the Victorian Government Department of Jobs, Precincts and Regions (DJPR) and the Royal Women’s Hospital in Melbourne, for their ongoing support in helping Navi develop medical innovations that will help children everywhere live brighter, healthier futures.