From finalising product development, to raising capital, to growing our team, this year has delivered key milestones in preparation for the Neonav ECG Tip Location Systems regulatory clearance and market entry.
Below are some of the important highlights of what has been an amazing 2023:
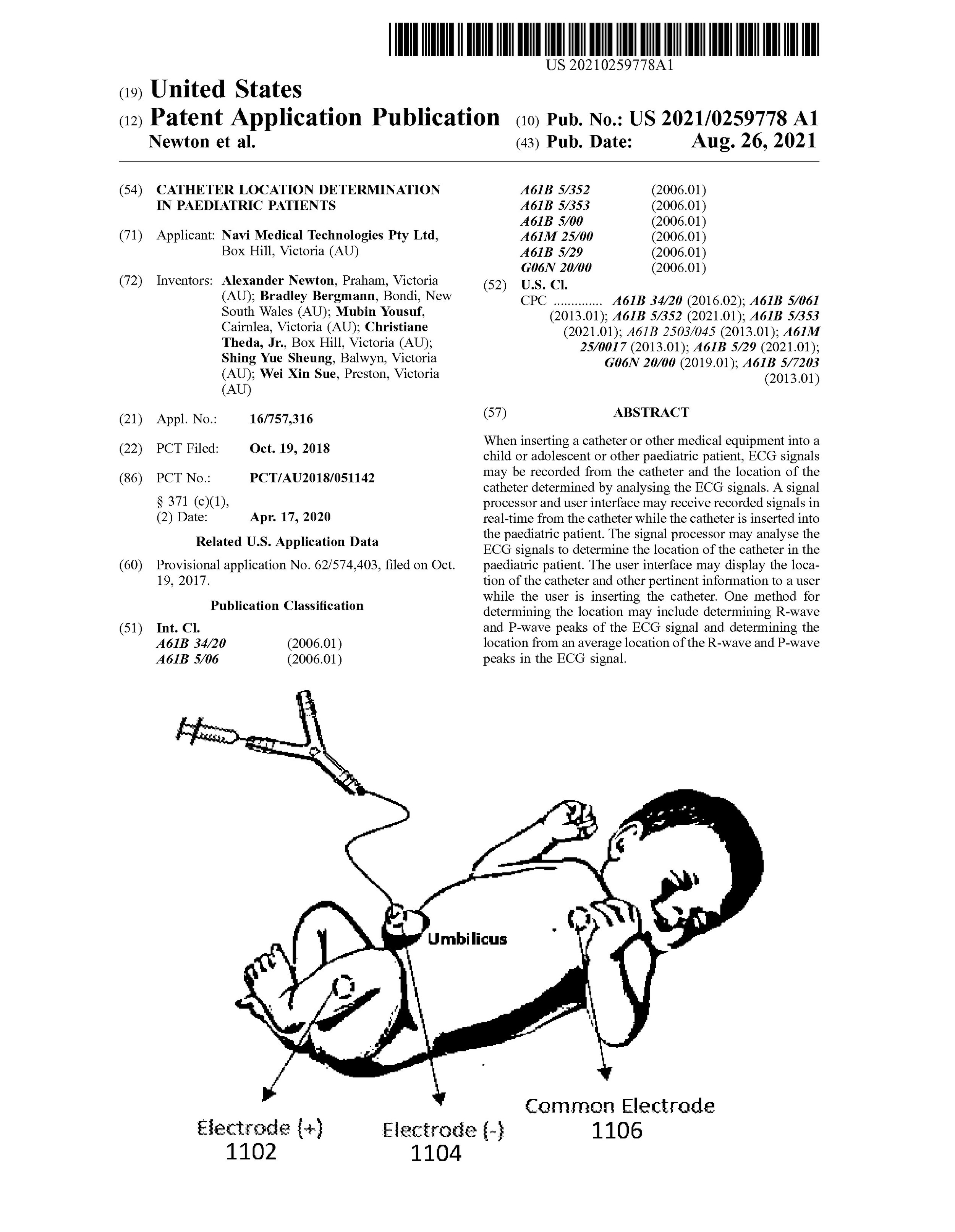
Completed The Neonav Product Development:
A significant objective for 2023 was to complete the product development work for the Neonav System in preparation for clinical trials in 2024.
After several prototype iterations, the Neonav Device design has been completed. The development of the device is a significant milestone for the company, and has been a huge, combined effort that included our internal engineering and regulatory teams, external product development partners, and the help of dozens of clinicians across the globe providing valuable user insight and feedback.
Pictured: 3D Render Of the Neonav ECG Tip Location System
Achieved Significant Clinical Milestones
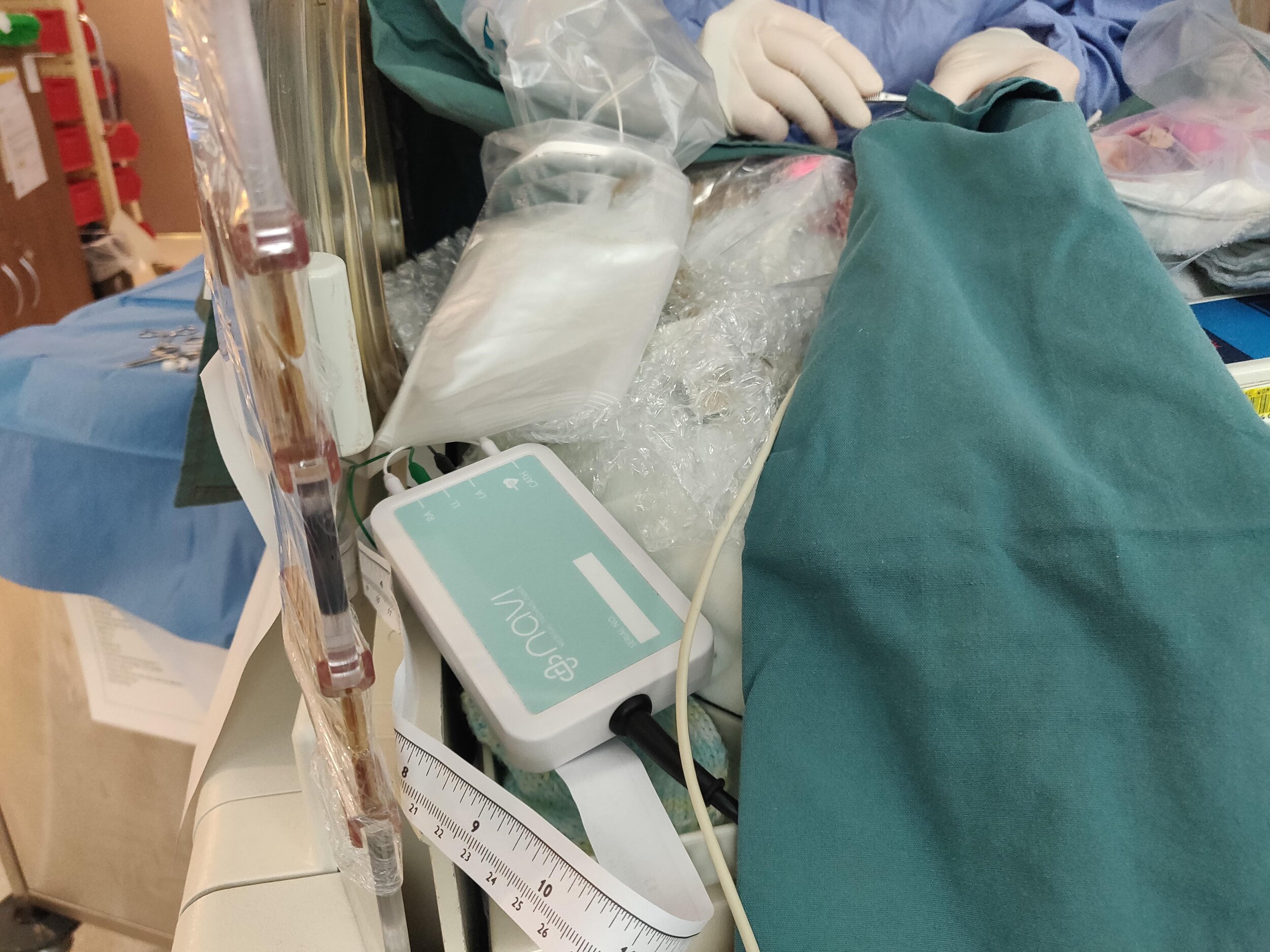
This year kicked off the Neonav “ECHO” study, where the Neonav device’s accuracy was compared to Ultrasound to determine how the device performs against the industry gold-standard in determining the location of Umbilical Venous Catheters (UVC’s) and Peripheraly Inserted Central Catheters (PICCs)
The results were extremely promising, with early indications showing a strong concordance between to both modalities.
Additionally, the clinical team conducted a number of studies to test various Neonav device hardware and software iterations, playing a crucial role in accelerating the Neonav development.
Investment & Grants:
During 2023, the our team was awarded a number of grants, with the highlight being the $2.3 million CRC-P grant funding. This funding was part of a $6.28 million project in collaboration with the University Of Melbourne, the Royal Women’s Hospital, and Design + Industry, and will support an upcoming pivotal clinical trial to generate data for FDA clearance.
In addition to the grant funding, Navi received an additional $1.34m from investors, which included a $700,000 lead investment from $2 billion investment fund Breakthrough Victoria
Patents Granted:
We now have 2 granted patents that cover the Neonav system in the USA and Japan, with several more regions soon to be granted. In addition, further work has progressed with other patent families to broaden the scope of our IP coverage.
Strategic partnerships
Navi formed a new strategic investment partnership with Breakthrough Victoria, which is a $2 billion investment fund. The announcement of the partnership took place at a media event at the Royal Women’s Hospital, which was attended by the Deputy Premier Ben Carroll (formally Minister of Innovation), and was featured on several TV and radio national news networks.
You can read more about Breakthrough Victoria’s investment in Navi here
Pictured: Navi team members with the Breakthrough Victoria team
Continued to grow our team:
During 2023, we were lucky enough to welcome some incredible people to our team, whos expertise in clinical, regulatory and quality affairs have provided invaluable contributions and insight.
Zorana Mayooran
An RA/QA Specialist who is instrumental in ensuring Navi meets all the necessary regulatory and quality requirements for FDA Clearance and beyond.
Rosina Velona
Clinical Research Coordinator who is managing a number of clinical activities both domestically and abroad as we seek FDA clearance and expand our commercialisation, research and development activities.
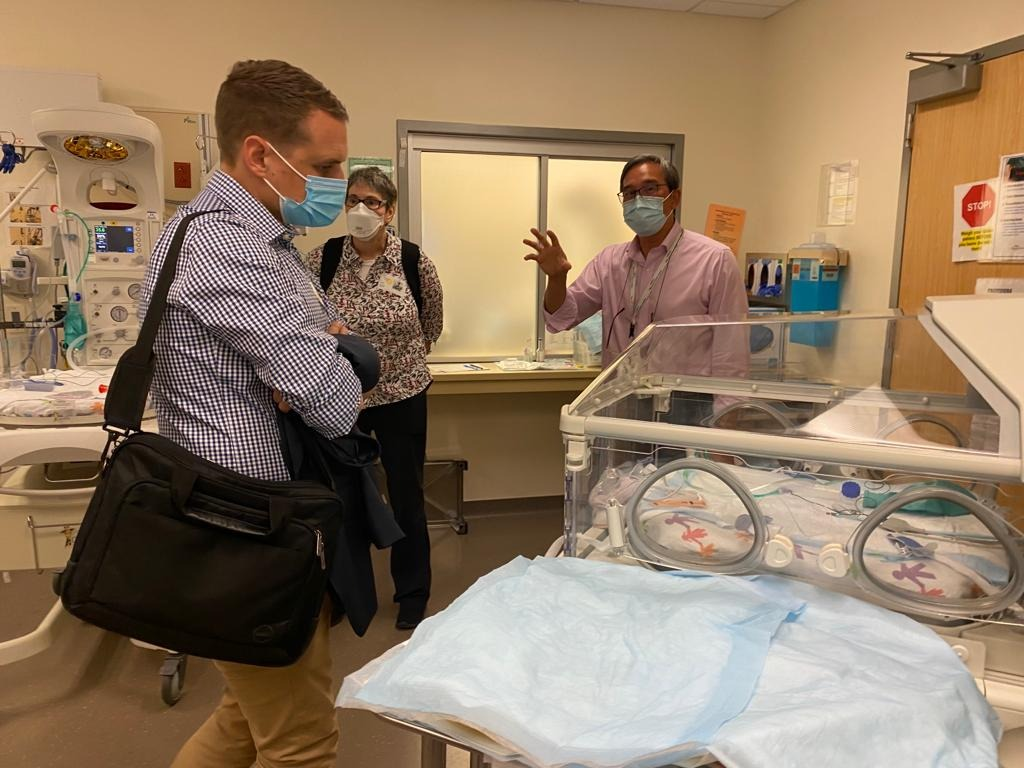
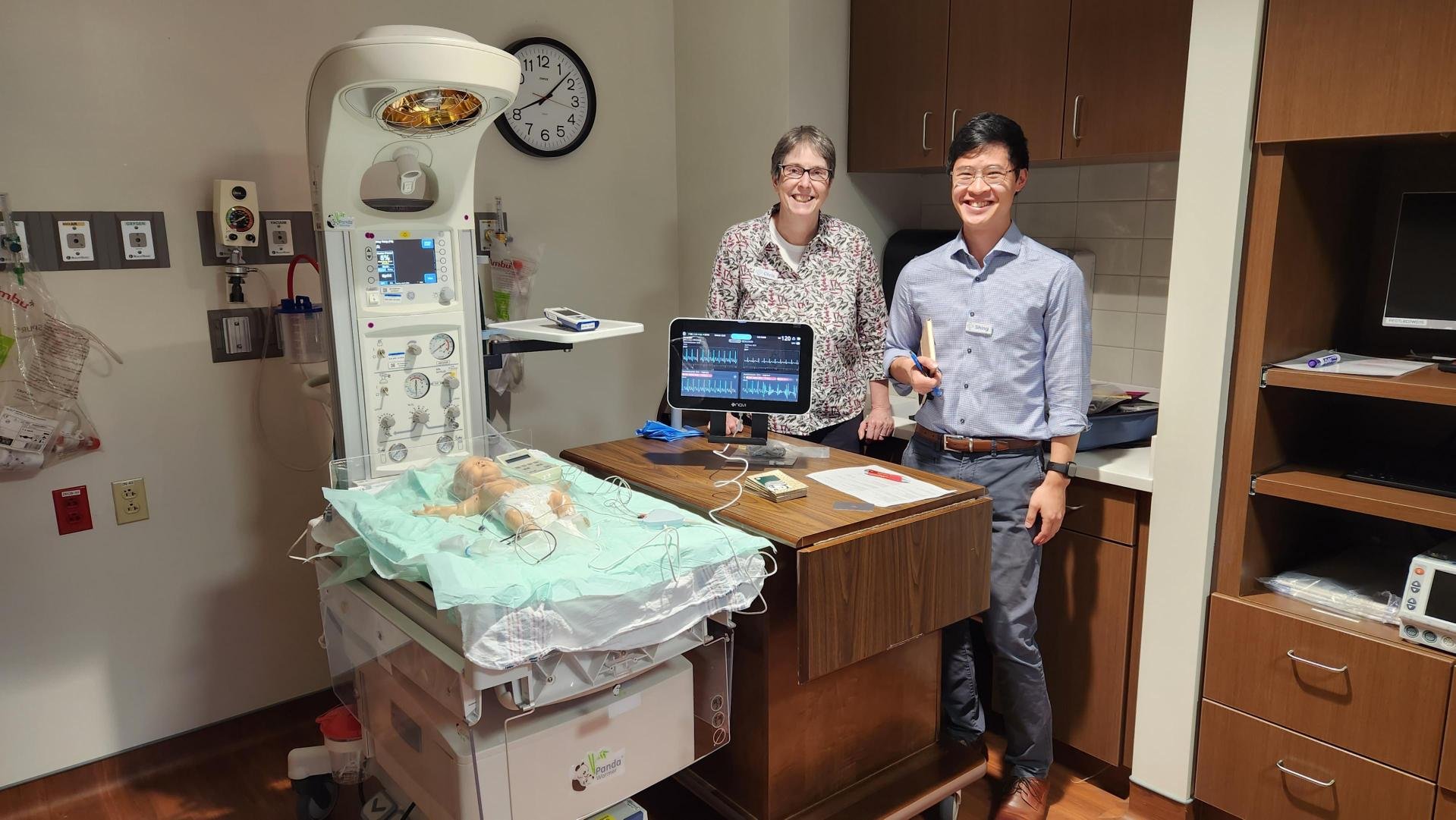
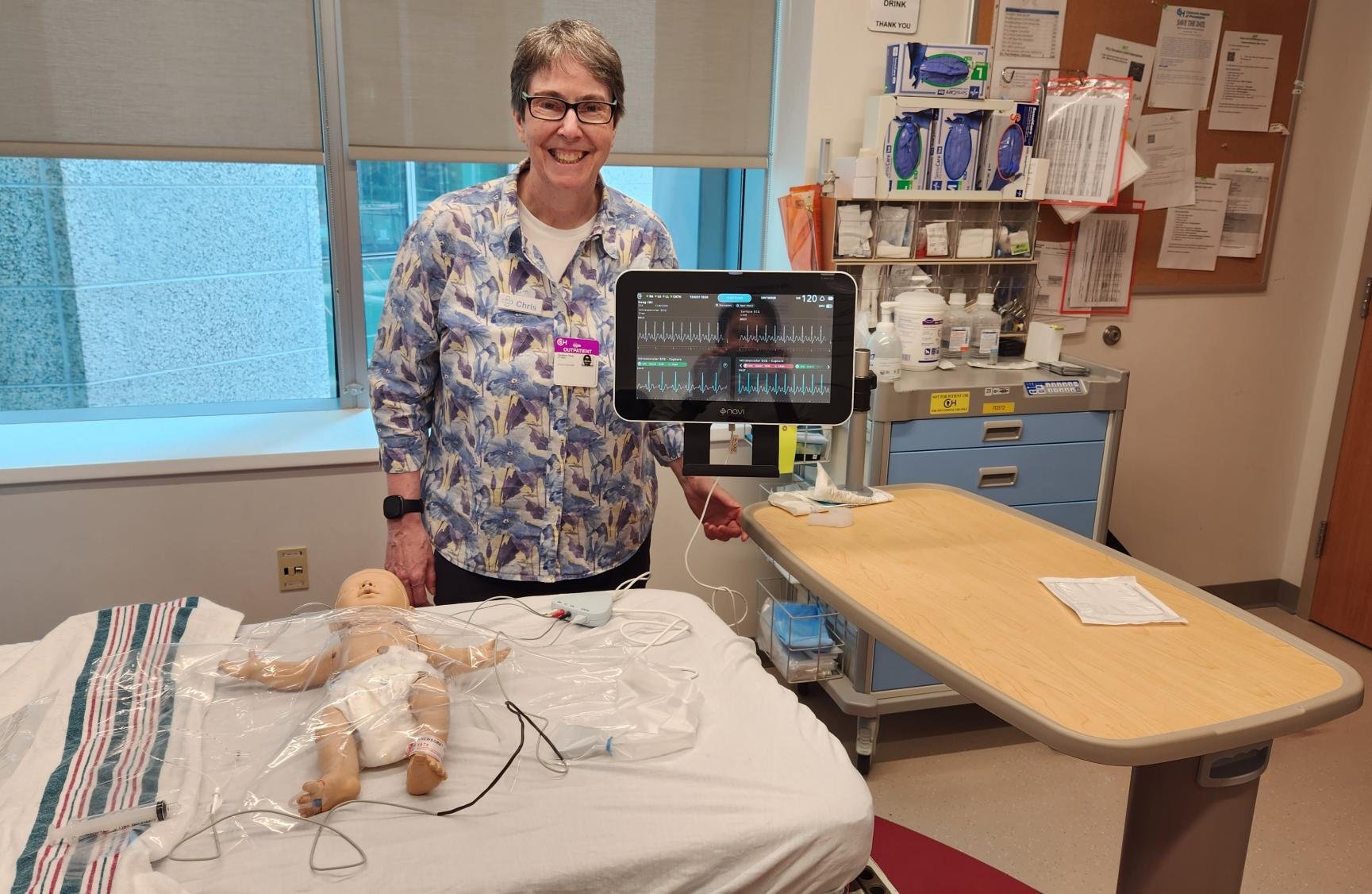
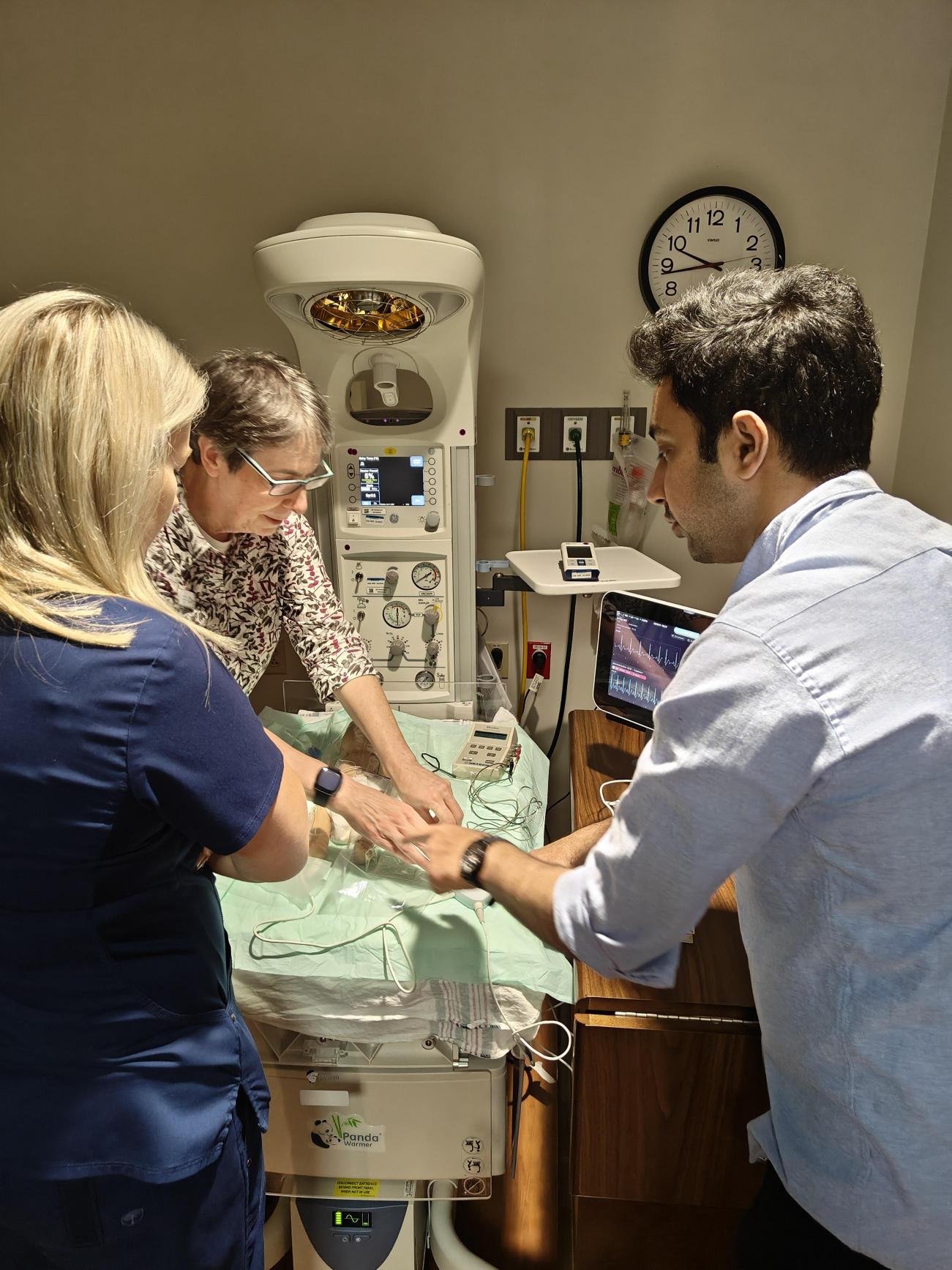
The Navi US Roadshow
Throughout the year, Navi team members spent several weeks in the United States visiting key hospital sites and talking with key opinion leaders in the vascular access industry to expand our network, gain critical insight into the US Children’s Hospital market, and receive important user feedback to help with the Neonav development.
The data collected from these trips have already been incorporated into the Neonav’s product design.







Navi CTO Recognised
Navi CTO and Co-Founder Mubin Yousuf was listed in the SAARI Collective's top 101 Startup Founders List!
This was in recognition for the incredible talent and innovation behind his work in developing the Neonav device. It's great to see his talent and expertise being recognised alongside an incredibly talented list of founders from the South Asian Australian community!
It has been a huge year for the Navi, and we would like to thank all our partners who have contributed their time, knowledge, capital and other resources in helping us achieve so many critical milestones in 2023, and to continue our mission to provide brighter, healthier futures for children everywhere in 2024.