Navi is pleased to announce the successful completion of its latest funding round, securing a total of $2.4 million. Investors who took part in this round of funding included a $700,000 lead investment from $2 billion investment fund Breakthrough Victoria, as well as federal grants from the Australian Government
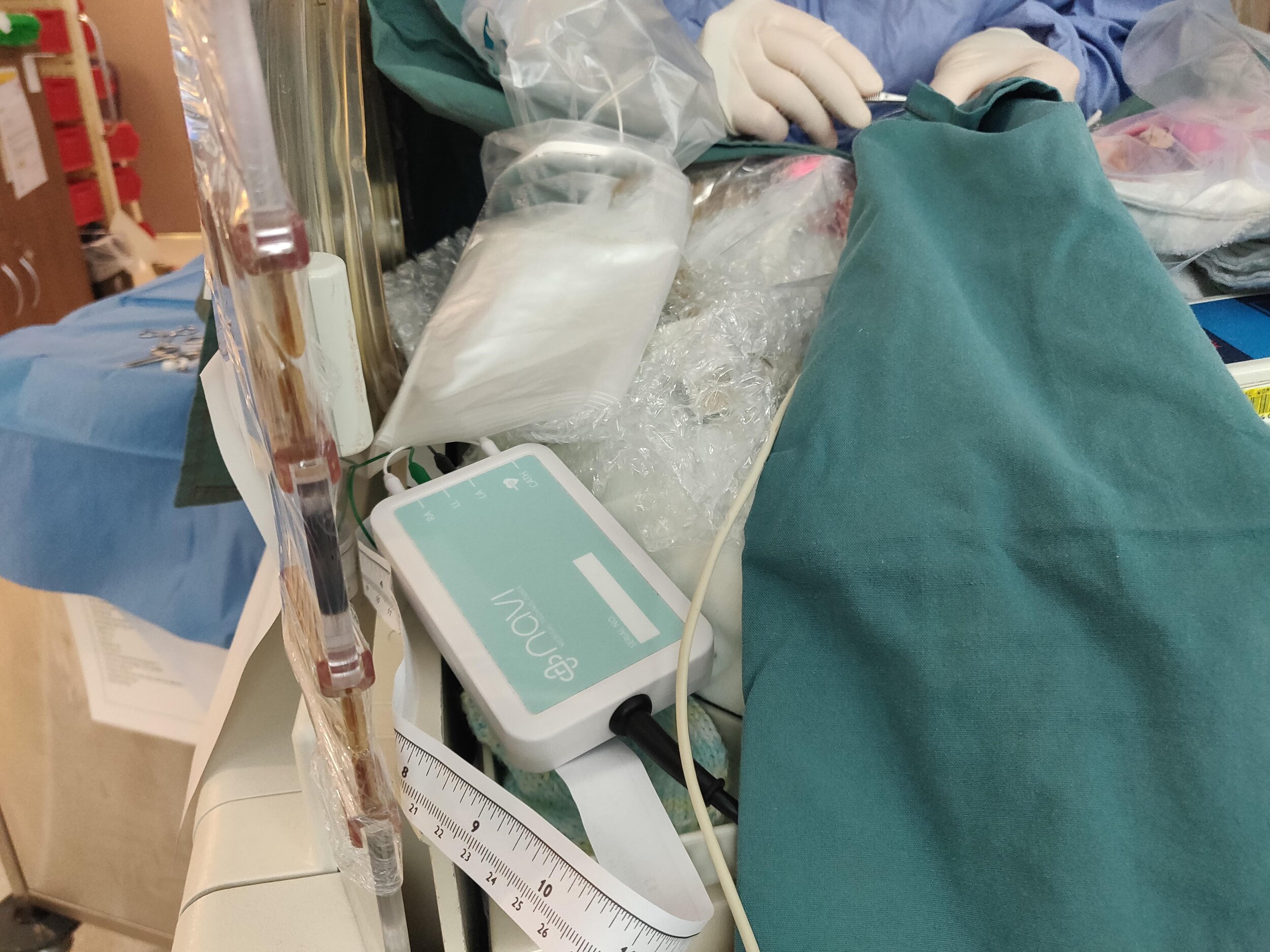
Navi is currently developing the Neonav® ECG Tip Location System, which is a novel medical device designated as a Breakthrough innovation by the US Food and Drug Administration (FDA).
Navi Medical Technologies CEO Alex Newton said the Neonav is set to raise the standard of care by enabling doctors and nurses to administer therapies more safely to critically ill newborns and pediatric patients.
"At present, central catheters are inserted into the veins of critically ill newborns and young children in a blind procedure. Doctors are only able to confirm the location of the catheter with an x-ray after the procedure has already taken place. Unfortunately, this approach leads to a large number of instances where the catheters are misplaced, exposing patients to potentially life-threatening complications if left undetected.”
Pictured (left to right): Navi CMO and co-founder Associate Professor Christiane Theda, Navi CEO and co-founder Alex Newton, Minister of Industry and Innovation Ben Carroll.
The Neonav system, which is currently in advanced stages of development, represents a transformative solution by seamlessly connecting to a standard catheter and capturing electrical signals from the patient’s heartbeat. Using a proprietary algorithm, the Neonav system provides real-time catheter location of the catheter tip. Real-time tip location allows clinicians to position the catheter quickly and safely during procedures, as well as enable ongoing surveillance of the catheter position to ensure safe continued use.
Pictured: Navi CMO Associated Professor Christiane Theda demonstrating the Neonav prototype to Minister of Industry and Innovation Ben Carroll
The Minister for Industry and Innovation Ben Carroll, who was present at today’s announcment at the Royal Women’s Hospital, reiterated the governments support for Navi and its technologies postiential impact, “We invest in innovation to maximise the economic benefits for Victoria and to ensure better care for our youngest patients.”
Alex Newton said funding will be channelled towards the next critical phase of work. “Primarily we’re focused on completing product development activities and a major clinical trial to obtain FDA approval, which will allow us to set up our manufacturing and logistics capabilities in preparation for our entry into the U.S market.”
Associate Professor Christiane Theda, Chief Medical Officer at Navi Medical Technologies, expressed her gratitude for the recent investment and emphasized the significance of this milestone, "For more than 30 years I’ve been dreaming of a device to assist with placement of catheters to help my patients. With the support of Breakthrough Victoria and our other partners, we’re now one big step closer to bringing the Neonav ECG Tip Location System to the bedside."
About Navi Medical Technologies:
Navi Medical Technologies is a medical device startup headquartered in Melbourne, Australia. Focused on delivering clinical innovations, Navi's passion lies in creating cutting-edge solutions that enable children worldwide to live brighter and healthier futures through its medical innovations. Navi is currently developing the Neonav ECG Tip Location System, designated as a Breakthrough device by the US FDA, and will enable doctors and nurses to provide better, safer care for critically ill newborn and pediatric patients.
For media inquiries, please contact:
Brad Bergmann
Director of Communications, Navi Medical Technologies
Phone: +61 (3) 9059 4919
Email: brad@navitechnologies.com