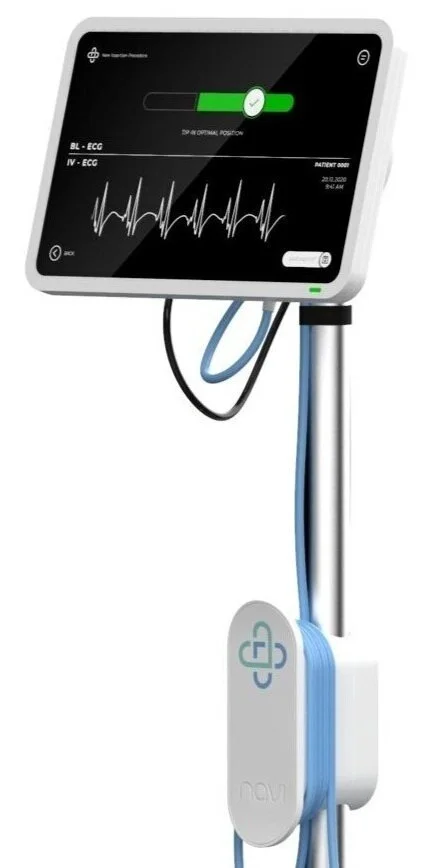
At Navi Medical Technologies, we believe there is a critical shortage in fit-for-purpose products for the hospitals and clinicians that care for critically ill newborns and children.
In fact, only around 15% of pediatric medical devices approved by the US FDA seek an indication for use in newborn patients. This means that less than 5% of all FDA approved medical devices have an indication for use in newborns; our most vulnerable patient cohort.
This forces pediatric clinicians to often ‘make do’ with devices and equipment designed for adults or larger patients, and are not optimised for the care of critically ill newborns or children.
We are determined to use our experience in working closely with world-leading healthcare providers to understand unmet clinical needs and make exceptional products that solve important clinical problems.